Open Access
Review
Table 4
Summary of functional groups, adsorption mechanisms, and representative materials for various radioactive nuclides.
| Target Ion | Functional groups/ligands | Adsorption mechanism | Representative materials |
|---|---|---|---|
| Sr2+ | Crown Ethers | The cavity size of crown ethers matches that of Sr2+ ions, enabling the formation of host–guest complexes. | Crown ether-functionalized silica gels and crown ether-grafted polymers. |
| Carboxylic Groups (–COOH) | Electrostatic attraction between negatively charged carboxylate groups and Sr2+ facilitates coordination bonding. | Carboxymethyl cellulose (CMC), poly acrylic acid (PAA). | |
Phosphate Groups (–PO3H₂/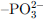 ) ) |
Strong ion exchange interactions between phosphate groups and Sr2+ lead to the formation of Sr–PO4 complexes. | Phosphate-functionalized mesoporous materials, zirconium phosphate composites. | |
| Cs+ | Calixarene Derivatives | The cavity size of calixarenes matches that of Cs+, enabling selective adsorption via hydrophobic interactions and electrostatic attraction. | Sulfonated calixarenes, calixarene-modified resins [4]. |
| Crown Ethers | Crown ethers exhibit strong coordination with Cs+, with enhanced selectivity at high pH. | Crown ether-grafted polymers, inorganic/organic hybrid crown ether materials. | |
| Phosphate Groups (–PO3H₂) | Cs+ is preferentially adsorbed via ion exchange due to the high affinity of phosphate groups for monovalent cations. | Titanium phosphate, zirconium phosphate-based ion exchange materials. | |
| Prussian Blue Analogs | Specific structures and functional groups exhibit spatial and electronic compatibility with Sr2+ ions. | Potassium cobalt/nickel/titanium/copper/cadmium ferrocyanides. | |
I−/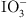 |
Quaternary Ammonium Groups (–NR3+) | Anion exchange occurs as the positively charged quaternary ammonium groups attract I−, forming ion pairs. | Strong-base anion exchange resins. |
| Amino Groups (–NH₂) | Protonated amino groups (–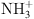 ) adsorb I− via electrostatic interactions or form hydrogen bonds with ) adsorb I− via electrostatic interactions or form hydrogen bonds with 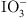 . . |
Chitosan, amino-functionalized mesoporous silica. | |
| Porous Materials Loaded with Ag+ | Ag+ reacts with I− to form insoluble AgI precipitates. | Silver-loaded zeolites, Ag@MOF composites. | |
| Co2+/Co3+ | Amino Groups (–NH₂) | Amino groups form coordination bonds with Co2+/Co3+, with polyamine structures enhancing chelation. | Polyethyleneimine (PEI), ethylenediamine-modified materials. |
| Thiol Groups (–SH) | Strong coordination between thiol groups and Co2+/Co3+ results in stable complex formation. | Thiol-functionalized silica, thiol-modified magnetic nanoparticles. | |
| Carboxylic Groups (–COOH) | Carboxylate groups chelate Co2+/Co3+ to form multidentate coordination structures. | Poly acrylic acid (PAA), carboxylic acid-functionalized MOFs. | |
| Dendritic Polyamines | Highly branched amine networks offer multiple coordination sites for selective Co2+/Co3+ adsorption. | PAMAM dendrimer-grafted nanomaterials |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

