| Issue |
Res. Des. Nucl. Eng.
Volume 1, 2025
|
|
|---|---|---|
| Article Number | 2025003 | |
| Number of page(s) | 19 | |
| DOI | https://doi.org/10.1051/rdne/2025003 | |
| Published online | 04 July 2025 | |
Review
Research advances on adsorption materials for the purification of radioactive wastewater
1
School of Naval Architecture and Maritime, Zhejiang Ocean University, Zhoushan 316022, China
2
State Key Laboratory of Water Environment Simulation, School of Environment, Beijing Normal University, Beijing 100875, China
* Corresponding author: h20091957@126.com
Received:
15
February
2025
Accepted:
1
May
2025
With the development of nuclear industry, the prevention and control of radioactive risks remains a critical challenge. Radioactive wastewater contains various radionuclides, such as 90Sr, 137Cs, and 239Pu, which pose threats to both the environment and human health. Effective treatment and purification of radioactive wastewater are therefore crucial. Adsorption methods, due to their efficiency and energy-saving characteristics, play a crucial role in radioactive wastewater purification technologies. This review summarizes the research progress on adsorption materials used for radioactive wastewater purification, including natural adsorbents, composite adsorbents, engineered adsorbents, adsorption membrane materials, and photothermal evaporation adsorbents. The advantages, disadvantages, and future prospects of these materials are analyzed. Studies indicate that adsorption materials hold great potential in the field of radioactive wastewater treatment, and however, issues such as low adsorption capacity, insufficient selectivity, and the risk of secondary contamination still remain. This paper reviewed the research advances of adsorption materials used for the purification of radioactive wastewater, and discussed their advantages, limitations, and future research directions.
Key words: Radioactive Wastewater / Adsorption Technology / Adsorption Materials / Radionuclides
© The Author(s) 2025. Published by EDP Sciences and China Science Publishing & Media Ltd.
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
With the development of nuclear industry, the prevention and control of radioactive environmental risks associated with radioactive contamination has become increasingly urgent. Radioactive wastewater produced by activities such as nuclear power plant operation, radioactive mineral mining, nuclear medicine procedures, uncontrolled nuclear testing, and accidental discharges of radioactive effluent poses a critical threat to ecosystems and human health [1]. Radioactive wastewater mainly contains radionuclides such as 90Sr, 137Cs, 239Pu, 131I, 99Tc, and 79Se [2]. Achieving efficient purification and recovery of radionuclides from radioactive wastewater is of crucial for the safe use of nuclear energy and mitigation of environmental risks related to radionuclide contamination. Radioactive wastewater purification technologies mainly include adsorption, membrane separation, electrochemical treatment, evaporation, precipitation, and phytoremediation [2]. Among these, adsorption has attracted growing attention due to its efficiency and energy-saving properties in radioactive wastewater purification, making it a research hotspot in recent years. The total research output of various purification technologies over the past decade is shown in Figure 1. The data were sourced from the Web of Science Core Collection. Adsorbent materials used for radioactive wastewater treatment primarily include natural adsorbents, composite adsorbents, and novel functionalized adsorbents. This paper reviewed the research progress of adsorption materials in radioactive wastewater purification, analyzing their advantages, limitations, and future application prospects.
 |
Fig. 1 Annual number of publications on radioactive wastewater treatment using adsorption, membrane technologies, electrochemical methods, evaporation systems, phytoremediation, and precipitation from 2014 to 2024. Data were obtained from the Web of Science Core Collection. |
2 Research progress of adsorption methods in the purification of radioactive wastewater
2.1 Natural adsorbents
Renewable natural resources, such as clay, minerals, and biopolymers, due to its abundant resources and low cost, it is widely used for the purification of radioactive wastewater [3]. For instance, clay minerals are considered ideal adsorbents due to their high availability, low production costs, non-toxicity, large specific surface area, excellent adsorption capacity, and strong ion exchange potential [4, 5]. Zeolite, a low-cost and easily accessible porous silicate material, exhibits exceptional adsorption capacity and ion-exchange properties due to its abundant pores and high surface area. These properties make zeolite widely used for adsorbing potentially toxic elements (PTEs) [6] and radioactive nuclides. For instance, El-Kamesh investigated the adsorption of 137Cs and 90Sr by zeolite [7]. Under conditions of 298 K and pH = 6, after 3 h of contact, the adsorption capacity of zeolites for 137Cs reached 77 mg/g, with a radionuclide removal efficiency of 95.66%. Under the same conditions, the adsorption capacity for 90Sr was 95 mg/g [8]. Meanwhile, Abdollahi et al. also studied the adsorption characteristics of natural zeolite for Cs-137 and 90Sr, and it was found that under pH = 7.23, the adsorption efficiency of natural zeolite for 137Cs was 67.8%, while at pH = 7.9, the adsorption efficiency for 90Sr reached 93.5% [9]. This suggests that pH is a key factor influencing the adsorption capacity of zeolites for radionuclides. Identifying the optimal pH conditions is crucial for enhancing the adsorption efficiency of zeolite [5].
Bentonite is a natural clay mineral with a high surface area and good cation exchange capacity, which enables it to provide numerous adsorption sites. The adsorption mechanism of natural bentonite materials for radioactive nuclides is illustrated in Figure 2a. According to the study by Wang et al. [10], pH and ion strength are critical factors influencing the adsorption capacity of bentonite for Uranium(VI) [U(VI)], with ion exchange playing a pivotal role in the adsorption process. The study also demonstrated that bentonite has considerable adsorptive capability for U(VI), with an adsorption capacity reaching 9.124 mg/g. In addition, Chen et al. studied the adsorption performance of natural bentonite for Eu(III) and found that its adsorption capacity depended on the pH of the solution, and the adsorption capacity of bentonite for Eu(III) can reach 4.52 mg/g, indicating that bentonite shows promising potential for treating Eu(III)-containing radioactive wastewater [11]. Moreover, bentonite minerals, as a natural adsorbent, exhibit excellent performance in treating radioactive wastewater containing 137Cs, achieving a removal rate of up to 98% [12]. The adsorption effect of bentonite, attapulgite, and kaolinite for 137Cs are shown in Figure 2b. As seen, besides bentonite, attapulgite and kaolinite also exhibit high adsorption capacities due to their unique internal surface areas and pore sizes. By comparing and analyzing the adsorption performance of two clay minerals, attapulgite and kaolinite, with other modified adsorbents for 137Cs, it was found that attapulgite exhibited exceptional adsorption efficiency, achieving a removal rate of up to 97%, while the removal efficiency of kaolinite was 75% [13]. In contrast to synthetic adsorbents, attapulgite and kaolinite clays serve as naturally occurring, economical, and eco-friendly adsorbents [13]. Montmorillonite is a hydrous aluminosilicate mineral with a layered silicate sheet structure, as shown in the cross-sectional model in Figure 2c, and this structure makes montmorillonite suitable for application in the treatment of radioactive nuclides [11]. Yılmaz et al. demonstrated that montmorillonite has excellent adsorption capacity for the radioactive isotope 177Lu, achieving a removal of approximately 90% of 177Lu and reaching adsorption equilibrium within 90 min [14]. Additionally, montmorillonite clay, as an abundant and sustainable adsorbent, is also suitable for treating various radioactive medical waste, such as 131I and Tc(99-m) [14].
 |
Fig. 2 (a) Main adsorption mechanisms of bentonite-based materials for radionuclides [11]; (b) (1): Adsorption capacity (qe) (2): Adsorption distribution coefficient (Kd), A: Attapulgite, B: Bentonite, K: Kaolinite [13]; (c) Cross-sectional model of montmorillonite layered silicate sheets [11]. |
Biological adsorbents, such as bacteria, fungi, algae, and plants, demonstrate excellent potential in the treatment of radioactive wastewater due to their efficient adsorption capacity, adaptability to specific pH values, and excellent selectivity and stability towards radioactive nuclides. For example, Takehiko et al. found that the adsorption capacity of Streptomyces for uranium (U) reaches 380 μmol/g [15]. Additionally, studies have shown that the red winter yeast, as a natural organic adsorbent, can achieve an adsorption efficiency of up to 95% for 137Cs at pH 6 and 25 °C [16]. Biomass adsorbent materials are promising candidates for radioactive nuclide adsorption due to their wide availability, strong renewability, and environmental friendliness. Furthermore, biomass-based adsorbents, with their exceptional high specific surface area and complex porous structure, provide abundant active sites for adsorption, resulting in a substantial increase in their adsorption efficiency. For instance, Ahmadpour et al. used almond shells as a biosorbent to remove radioactive strontium (Sr2+), achieving an adsorption capacity of 116.3 mg/g [17]. Additionally, leaves exhibit promising effectiveness in the removal of radioactive wastewater. For example, Benalia et al. used unmodified oak leaf powder to directly treat strontium-containing simulated wastewater. Under neutral conditions, oak leaves demonstrated good adsorption performance, with an adsorption rate exceeding 80% [18]. Moreover, Sharma et al. studied the adsorption performance of poplar leaves for the removal of radioactive thorium (Th(IV)) and found that after alkaline modification, the surface-negative functional groups of poplar leaves increased, thereby enhancing their adsorption efficiency for thorium ions [19]. Overall, in the past few years, the efficacy and properties of natural adsorbents in capturing radioactive isotopes have garnered extensive interest. The performance and characteristics of various types of natural adsorbents for wastewater purification are summarized in Table 1. Although natural adsorbent materials exhibit potential in the purification of radioactive nuclides, their adsorption capacity is generally lower than that of the composite adsorbent materials.
Performance and characteristics of natural adsorption materials applied in radioactive wastewater purification.
2.2 Composite adsorbents
Due to the common drawbacks of natural adsorbent materials, such as low adsorption capacity, poor selectivity, and poor regeneration performance, researchers have turned their attention to composite adsorbents. Composite adsorbents refer to materials synthesized by chemical or physical methods that exhibit specific adsorption properties. These materials can be designed with tailored pore structures, surface functional groups, and chemical compositions to achieve efficient adsorption of specific gases, liquids, or solid pollutants. For example, potassium hexacyanoferrate (PBAFe) [21], silver-loaded activated carbon fiber composites (Ag@ACF) [22], and carboxymethyl chitosan gel titanium phosphate (TiP) aerogels [23], these materials typically have a high specific surface area and pore volume, providing more adsorption sites to achieve higher adsorption capacities. Additionally, through molecular design, they can achieve high selectivity for specific pollutants, reducing adsorption of other substances. Many composite adsorbents exhibit good chemical and thermal stability, maintaining their adsorption capacity under variable environmental conditions. Furthermore, they can be regenerated through simple physical or chemical methods, restoring their adsorption properties for repeated use. For example, alginate@polymeric beads (alg@PB) exhibits good adsorption stability under various pH conditions, particularly at pH 10, indicating that it has good adsorption performance across a wide pH range [24]. In contrast, the adsorption performance of citric acid-activated magnesium-aluminum-iron composite (CAA@MgAlFe) is critically influenced by pH [25]. Under acidic conditions (pH 2–4), the adsorption capacity is low due to the dissolution effect of layered double hydroxides (LDHs). Under neutral to mildly alkaline conditions (pH 4–10), the adsorption capacity for uranium decreases with increasing pH, limiting its application range in different pH environments. The preparation process and adsorption mechanism diagram of CAA@MgAlFe are shown in Figures 3 and 4.
The adsorption targets, conditions, performance, and characteristics of composite materials in the purification of radioactive wastewater are shown in Table 2. It can be observed that both Ag@ACF and CAA@MgAlFe composite materials achieve their maximum adsorption capacities after hydrothermal treatment. Chen et al. [22] synthesized Ag@ACF composite materials by using hydrothermal modification and vacuum reduction techniques, which allowed for the uniform distribution of silver nanoparticles on the surface and in the pores of the activated carbon fibers (ACF), providing a large number of active sites for iodine ions. On the other hand, after hydrothermal treatment [25], the combination between layered double hydroxides (LDHs) and calcium alginate gel in the CAA@MgAlFe composite material became more compact, forming a more stable composite structure. Under high-temperature conditions, the crystal structure of LDHs was further refined, and the connections between particles became stronger. This not only improved the mechanical strength and durability of the material but also made it less prone to structural damage or particle detachment under complex environmental conditions, thus extending the material’s lifespan.
Performance and characteristics of composite adsorption materials applied in radioactive wastewater purification.
The preparation of composite adsorbent materials is a challenging and complex process, involving the selection of various materials, optimization of synthesis methods, and tuning of performance. Precise control of the material’s microstructure is crucial to ensure that the prepared composite materials exhibit favorable adsorption performance under various environmental conditions. Li et al. [21] developed a Fe-Co framework Prussian Blue Analogue (PBA) adsorbent–potassium ferrocyanide cobalt (PBAFe)–using a simple chemical synthesis method, which effectively adsorbs cesium ions. The adsorption mechanism of PBAFe for Cs+ is shown in Figure 5. The material demonstrates excellent adsorption stability and selectivity under mildly acidic conditions. In practical applications, it can efficiently remove Cs+ in complex water environments, with a removal efficiency of up to 83.59%. On the other hand, Ye et al. [26] developed hollow porous composite nanofibers (HPN) that enhance the interface exposure of nanocatalysts through electrospinning and phase separation techniques, which effectively reduces mass transfer resistance, enabling rapid adsorption and efficient reduction of uranium. The uranium removal mechanism is shown in Figure 6, with removal efficiency reaching 98.86% within 4.5 h. However, the preparation of these materials typically requires precise synthesis conditions and complex process control (the preparation process of HPN is shown in Fig. 7). Researchers combine different types of adsorbent materials to leverage the unique advantages of each material and utilize their synergistic effects to achieve efficient removal of pollutants. While enhancing the adsorption capacity of composite materials, attention should be given to the development of regeneration technologies. Promoting research into efficient material regeneration techniques can enable the reuse of adsorption materials, reduce operational costs, and minimize potential secondary environmental risks.
2.3 Engineered adsorbents1
Engineered adsorbents refer to materials whose adsorption functionality is artificially tailored through structural design, surface modification, or nanoscale engineering, with performance governed by human intervention rather than the intrinsic properties of raw components. The materials of metal-organic frameworks (MOFs), covalent organic frameworks (COFs), MXenes and graphene have demonstrated substantial potential for the treatment of radioactive wastewater. MOFs and COFs have become widely studied due to their high specific surface areas and tunable porous architectures, which allow for the rational design of adsorption behavior. Post-synthetic modifications, including targeted surface functionalization, can further increase their adsorption capacity by introducing specific binding sites. MXenes exhibit excellent electrical conductivity and hydrophilicity, features that make them highly effective in capturing heavy metal ions and organic pollutants when combined with deliberate surface engineering. Similarly, graphene offer exceptional mechanical strength and chemical stability, and its surface chemistry can be engineered to improve affinity toward targeted contaminants in complex wastewater matrices.
Currently, research on the engineered adsorbent materials focuses on developing new synthesis strategies to enhance their adsorption efficiency, selectivity, and long-term stability. The PPFM composite material, composed of nitrogen-rich fibers as the inner layer and porous Zr-MOFs as the outer layer [30], exhibits excellent performance in iodine adsorption and the degradation of the nerve agent simulant DMNP due to its unique core-shell structure. The dual functionality of the PPFM composite material in treating toxic compounds is illustrated in Figure 8. This material demonstrates a high iodine adsorption capacity of 609 mg/g without the need for additional co-catalysts, and can eliminate 80% of DMNP within 7 min, indicating rapid response and high efficiency. Furthermore, MOF materials also exhibit outstanding performance in removing Co(Ⅱ) [31]. The UiO-66-Lys/PAN nanofiber membrane, prepared via electrospinning, shows a pure water flux of 872 Lm−2 h−1 and a Co(Ⅱ) retention rate of 45.4%, with a maximum adsorption capacity reaching up to 41.4 mg/g, along with good radiation stability [31].
COFs and their composites show broad application prospects in addressing radioactive wastewater. Yang et al. focused on the simultaneous removal of 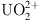 and
and 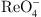 using electrostatic adsorption technology [32]. Their study found that COF-1 and COF-2 exhibited exceptionally high adsorption capacities, which were 2.5 times and 2.1 times higher, respectively, than those achieved by physicochemical adsorption methods. Li et al. highlighted the extensive application potential of COFs in water treatment, providing an overview of the classification and synthesis methods of COFs [33], and summarizing the development history of COFs, as detailed in Figure 9.
using electrostatic adsorption technology [32]. Their study found that COF-1 and COF-2 exhibited exceptionally high adsorption capacities, which were 2.5 times and 2.1 times higher, respectively, than those achieved by physicochemical adsorption methods. Li et al. highlighted the extensive application potential of COFs in water treatment, providing an overview of the classification and synthesis methods of COFs [33], and summarizing the development history of COFs, as detailed in Figure 9.
Currently, research on MXene materials found that MXene had favorable adsorption performance for Eu(III) [34]. The synthesis process and structural characteristics of MXene are shown in Figure 10. MXene was modified by citric acid and alkali treatment to form CA-Alk-Ti3C2Tx [35], and the structure of the TiO2/MXene composite material is shown in Figure 11. Under optimized conditions, this material exhibits a maximum adsorption capacity of 117.87 mg/g for Eu3+, demonstrating high adsorption efficiency and good reusability. Liu et al. outlined the characteristics and properties of MXene materials and provided a comprehensive summary of the research progress on MXenes, and they also proposed potential future research directions to address the challenges MXene materials face in practical applications [34].
In the field of uranium adsorption, graphene materials after modification have shown promising application potential. Li’s group used density functional theory (DFT) simulations to investigate the adsorption performance of single-side fluorinated graphene (ssFG) [36]. The findings revealed that the ssFG exhibits a strong adsorption capacity for uranium atoms, particularly the C2F structure, which demonstrates a high adsorption energy of 3.45 eV. Shao et al. successfully developed a graphene electrode modified with amidoxime groups (PAO-N-rGO/CF), which achieves efficient adsorption of radionuclides through chemical bonding between the amidoxime groups and U(Ⅵ). The study found that under specific conditions, the removal efficiency for U(VI) reached 99.93%, with excellent cycling stability [37].
Carbon nanotubes (cCNTs) have become a subject of widespread interest for their potential in purifying nuclear wastewater. The cCNTs show remarkable adsorption efficiency for Co2+ ions, which is attributed to their large specific surface area and optimal isoelectric point, with a maximum adsorption capacity of 25.1 mg/g. The adsorption capacities of cCNTs for different metal ions, as well as a comparison with other functionalized carbon nanotubes (fCNTs), are presented in Figure 12 [38]. Meanwhile, the Fe3O4/CNT nanocomposite has shown outstanding adsorptive properties for U(VI), reaching a theoretical maximum adsorption capacity of 287.53 mg/g [39].
The performance and features of engineered adsorbent materials applied in radioactive wastewater purification are shown in Table 3, and it can be found that the engineered adsorbent materials have favorable adsorption performance broad application prospects for in radioactive wastewater treatment. The future research is suggested to focus on performance optimization, cost reduction, and preparation processes simplification.
Performance and characteristics of engineered adsorption materials applied in radioactive wastewater purification.
The key functional groups, adsorption mechanisms, and representative materials employed for the sequestration of four classes of radioactive nuclides (Sr2+, Cs+, I−/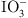 , and Co2+/Co3+) are summarized in Table 4. Ligand selection may be optimized according to ionic radius, charge, and coordination preferences. For Sr2+ and Cs+, crown ethers and phosphate moieties facilitate host–guest complexation and ion exchange to form M–PO4 linkages, while carboxylate groups further enhance Sr²+ uptake via electrostatic attraction and chelation. Adsorption of Co2+/Co3+ relies predominantly on polyamine and thiol functionalities forming stable coordination complexes. Anionic species (I−/
, and Co2+/Co3+) are summarized in Table 4. Ligand selection may be optimized according to ionic radius, charge, and coordination preferences. For Sr2+ and Cs+, crown ethers and phosphate moieties facilitate host–guest complexation and ion exchange to form M–PO4 linkages, while carboxylate groups further enhance Sr²+ uptake via electrostatic attraction and chelation. Adsorption of Co2+/Co3+ relies predominantly on polyamine and thiol functionalities forming stable coordination complexes. Anionic species (I−/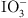 ) are sequestered through anion exchange using quaternary ammonium or protonated amine groups or by AgI precipitation on Ag+-loaded substrates.
) are sequestered through anion exchange using quaternary ammonium or protonated amine groups or by AgI precipitation on Ag+-loaded substrates.
Summary of functional groups, adsorption mechanisms, and representative materials for various radioactive nuclides.
The equilibrium adsorption capacities of Sr2+ across natural, composite, and engineered adsorbents exhibit a distinct increasing trend from low to high. Natural adsorbents generally demonstrate low capacities, typically within the range of 5–20 mg/g. Composite adsorbents show improved performance, with capacities predominantly ranging from 10–105 mg/g. Engineered adsorbents perform the best, with most samples exceeding 100 mg/g, and several reaching values between 200 and 320 mg/g, as illustrated in Figure 13. Concurrently, adsorption performance variability increases with material-design complexity, primarily owing to increased specific surface area, optimized pore architecture, and synergistic interactions among multiple phases, all of which enhance adsorption efficiency. These findings indicate that composite and engineered modifications can significantly enhance the Sr2+ removal efficiency of adsorbents, offering a strong rationale for the development of next-generation high-performance adsorption materials.
 |
Fig. 13 Comparison of equilibrium adsorption capacities of Sr2+ among natural, composite, and engineered adsorbents. Data were obtained from the Web of Science Core Collection. |
2.4 Adsorption membrane materials
Adsorption membranes are typical composite membranes, which combine the advantages of traditional adsorption methods and filtration technology, allowing for the design of membranes targeted at specific pollutants, demonstrating high selectivity in the adsorption of radionuclides [41]. The performance and features of adsorption membranes in radioactive wastewater treatment are shown in Table 5. Compared to traditional separation techniques, adsorption membrane technology does not require high temperature or pressure for pollutant removal, thereby reducing energy consumption. Once saturation is reached, the membrane material can be regenerated and recycled through a desorption process.
Performance and characteristics of adsorption membrane materials applied in radioactive wastewater purification.
Metal-Organic Frameworks (MOFs) have become a research focus in the development of adsorption membranes. For example, Long et al. [41] developed a PVDF/ZIF-8 nanocomposite adsorptive membrane, which uses reverse diffusion to precisely control the crystal growth on the metal ion side of the membrane. The PVDF/ZIF-8 exhibited efficient and durable iodine ion adsorption with a maximum adsorption capacity of 73.33 mg/g. The preparation process of the composite membrane and the mechanism for iodine removal from water are shown in Figure 14.
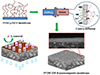 |
Fig. 14 Preparation process of PVDF/ZIF-8 nanocomposite membrane and schematic diagram of iodine removal from water [41]. |
Polypyrrole-based materials utilize the redox properties of polypyrrole to impart controllable surface charge to the membrane. Chen’s team [42] developed a microfiltration membrane adsorbent material for the rapid and efficient adsorption of radioactive nuclides. This micro-membrane material is composed of zeolitic imidazolate framework-67 (ZIF-67) nanoparticles combined with polyamide microporous membranes (PA), which was prepared using the permeation method and in situ synthesis. In batch adsorption experiments, the ZIF-67@PA microfiltration adsorption membrane demonstrated exceptionally high adsorption efficiency for thorium ions (Th(IV)), with a saturation adsorption capacity of 703.59 mg/g within 50 min, and good reusability, indicating its great potential for industrial applications. In ceramic membrane materials, Janus electro-controlled adsorption membranes can be designed by constructing functional layers on both sides of the membrane. Xiao et al. [43] firstly utilized microporous ceramic membranes to assemble two-dimensional (2D) imidazolate framework-L (ZIF-L) for the preparation of ZIF-L@SiC membrane (Fig. 15), which can effectively remove iodine. Under optimal flux and concentration conditions, the iodine removal efficiency approached 100%, with the iodine adsorption process shown in Figure 16. This study clearly demonstrates the strong selective adsorption capability of ceramic membranes for iodine.
Similarly, using the seed-assisted secondary growth method, Xu et al. [44] developed a columnar double-walled MOF adsorption membrane made of lac-Zn, with its preparation process and dynamic adsorption shown in Figure 17. The lac-Zn polycrystalline film exhibited excellent stability and strong adsorption capacity in irradiation experiments, with an iodine adsorption capacity of up to 755 mg/g in cyclohexane solution. In contrast, its dynamic adsorption capacity for iodine ions in aqueous solution was relatively weaker, at 31.72 mg/g. The adsorption efficiency of lac-Zn membranes for iodine is not only influenced by pore size and specific adsorption interactions, but also by membrane permeability, mass transfer rate, ion diffusion pathways, and separation time.
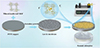 |
Fig. 17 Schematic diagram of the preparation process and dynamic adsorption process of lac-Zn columnar double-walled MOF adsorption membrane [44]. |
Adsorption membranes can also be used for radioactive wastewater treatment through simple filtration methods. This approach not only retains the high efficiency of adsorbents and filtration technology in purifying radioactive wastewater, but also enables the separation and recovery of low-cost powder adsorption materials, making it suitable for large-scale and high-repetition industrial applications. Cheng et al. [45] prepared a nickel-metal oxide-graphene oxide composite membrane (GO/Ni-MOF) using electrostatic self-assembly technology. The schematic diagram of the adsorption mechanism of GO/Ni-MOF for Sr2O3 is shown in Figure 18. Under optimal conditions, the maximum adsorption efficiency for Sr2O3 reached 32.99%, with an adsorption capacity of 72 mg/g. Additionally, electrochemical-switching ion extraction (ESIE) technology, which primarily utilizes electrochemical methods to control the adsorption and desorption processes of ions, enables efficient separation and purification of target ions [46]. Luo’s team [47] investigated a highly selective BiOI membrane based on Electrochemical Surface Ion Exchange (ESIE) technology. By triggering the formation of iodine vacancies on the adsorptive membrane through electrochemical reactions, this membrane exhibits high selectivity for iodine ions due to the ion vacancy trapping effect. Even in the presence of many competing anions, the membrane maintains a high iodine ion adsorption capacity of 328.3 mg/g. Furthermore, the iodine vacancy BiOI membrane maintains high extraction efficiency and stability, while allowing for easy desorption of captured iodine ions without introducing secondary environmental risks. The mchematic diagram of iodine vacancy BiOI film for adsorption and extraction of iodine ions from solutions containing radioactive elements is shown in Figure 19.
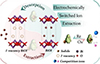 |
Fig. 19 Schematic diagram of iodine vacancy BiOI film for adsorption and extraction of iodine ions from solutions containing radioactive elements [47]. |
The application of adsorption membrane materials combines the multiple advantages of adsorption and membrane filtration methods, enabling efficient removal of radioactive nuclides while maintaining excellent adsorption capacity in highly saline radioactive waste solutions. Some adsorption membranes show a marked increase in adsorption capacity compared to pure adsorbents of the same material and can recover valuable elements such as uranium, enabling resources recycling. After saturation, adsorption membranes can restore their adsorption capacity through appropriate regeneration processes, enabling the repeated use of the membrane material, which reduces the treatment costs.
2.5 Evaporation adsorption materials
Traditional radioactive wastewater purification technologies mainly focus on the enrichment of radioactive nuclides, while the reduction of wastewater volume is equally important. Therefore, there is an urgent need for an efficient treatment method that can simultaneously reduce wastewater volume and enrich nuclides. Solar-driven interfacial evaporation is an effective treatment method that can reduce wastewater volume and concentrate nuclides, providing benefit of energy saving. Yu et al. synthesized a three-dimensional porous structural monomer sponge (KGS) using konjac glucomannan (KGM) and reduced graphene oxide (rGO), which exhibited excellent evaporation performance under single sunlight exposure. The preparation process of KGS is shown in Figure 20a, and the SEM images of the porous structure of KGS are shown in Figures 20b–20d. KGS exhibited a rapid evaporation rate of 1.60 kg/m2 h and an interfacial water evaporation efficiency of up to 92%. This performance is attributed to its outstanding absorbance, photothermal properties, thermal insulation, and efficient water transport. Additionally, the purification process reduced the concentration of radioactive elements (strontium, cesium, and uranium) in wastewater. KGS also exhibited outstanding durability and stability, with no noticeable decline in performance even after 20 cycles. These results indicate that KGS, utilizing solar-driven interfacial evaporation, can effectively treat radioactive wastewater and concentrate radioactive nuclides [52]. Solar-driven light evaporation adsorption materials have broad application prospects in the field of radioactive wastewater purification due to their energy efficiency, environmental friendliness, and ease of operation.
 |
Fig. 20 (a) Schematic diagram of the preparation process of KGS; (b–d) SEM images of KGS porous structures with different cations [52]. |
3 Conclusion
This review summarizes the current research status and characteristics of adsorption methods in the field of radioactive wastewater treatment, providing an in-depth analysis of the advantages and disadvantages of various adsorption materials. As one of the most important technologies for treating radioactive wastewater, adsorption methods have achieved numerous groundbreaking research results in the field of radioactive wastewater purification. In the future, to further promote the coordinated and sustainable development of environmental protection and the safe use of nuclear energy, further researches are still needed in the following areas:
As for natural adsorbents, their adsorption capacity and selectivity should be further enhanced through modification and activation methods.
Composite adsorbents and engineered adsorbents offer advantages such as high adsorption capacity and strong selectivity, but further research is needed to reduce preparation costs and simplify the preparation processes.
Adsorption membrane materials combine the advantages of traditional adsorption methods and advanced membrane technologies, demonstrating high selectivity in purifying radioactive nuclides. It is important to further enhance the adsorption capacity and recyclability of adsorption membranes.
Novel light evaporation adsorption materials provide notable energy savings and minimize the risk of secondary environmental impacts. However, the purification mechanisms of light evaporation adsorption materials for radioactive nuclides require further investigation. Kinetic and thermodynamic studies could be used to further explore the mechanisms of the purification process.
Funding
This research received no external funding.
Conflicts of interest
The authors declare no conflict of interest.
Data availability statement
No data are associated with this article.
Author contribution statement
Gao Junkai: Writing – Original Draft, Integration and Revision of the manuscript. Lv Anyuan: Writing – Section 2.4 “Adsorption Membrane Materials”. Zhao Ziru: Writing – Section 2.3 “Engineered Materials”. Luo Xiaoyue: Writing – Section 2.5 “Evaporation Adsorption Materials”. Li Zhaoyu: Writing – Section 2.2 “ Composite Absorbents”. Wang Huimin: Writing – Section 2.1 “Natural Absorbents”. Wang Runjie: Data Curation, Visualization, Figure Preparation. Chen Yan: Review and Editing. Hou Li’an: Supervision, Conceptualization, Methodology.
References
- H. Ma, M. Shen, Y. Tong, X. Wang, Radioactive wastewater treatment technologies: a review. Molecules 28, 1935 (2023). https://doi.org/10.3390/molecules28041935. [Google Scholar]
- H. Liu, T. Fu, M.T. Sarwar, H. Yang, Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. Appl. Clay Sci. 232, 106796 (2023). https://doi.org/10.1016/j.clay.2022.106796. [Google Scholar]
- D. Kadadou, E.A. Said, R. Ajaj, S.W. Hasan, Research advances in nuclear wastewater treatment using conventional and hybrid technologies: Towards sustainable wastewater reuse and recovery. J. Water Process Eng. 52, 103604 (2023). https://doi.org/10.1016/j.jwpe.2023.103604. [Google Scholar]
- G.D. Yuan, et al., Clays and clay minerals for pollution control. Develop. Clay Sci. 5, 587–644 (2013). [Google Scholar]
- W.A. Muslim, S.K. Al-Nasri, T.M. Albayati, H.Sh. Majdi, Treatment of actual radioactive wastewater containing Cs-137 using kaolinite clay minerals as eco-friendly adsorbents. Desal. Water Treat. 307, 162–170 (2023). https://doi.org/10.5004/dwt.2023.29908. [Google Scholar]
- O. Pourret, A. Hursthouse, It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int. J. Environ. Res. Public Health 16, 4446 (2019). https://doi.org/10.3390/ijerph16224446. [Google Scholar]
- A.M. El-Kamash, Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater. 151, 432–445 (2008). https://doi.org/10.1016/j.jhazmat.2007.06.009. [Google Scholar]
- I. Liaquat, R. Munir, N.A. Abbasi, B. Sadia, A. Muneer, F. Younas, M.F. Sardar, M. Zahid, S. Noreen, Exploring zeolite-based composites in adsorption and photocatalysis for toxic wastewater treatment: Preparation, mechanisms, and future perspectives. Environ. Pollut. 349, 123922 (2024). https://doi.org/10.1016/j.envpol.2024.123922. [Google Scholar]
- T. Abdollahi, J. Towfighi, H. Rezaei-Vahidian, Sorption of cesium and strontium ions by natural zeolite and management of produced secondary waste. Environ. Technol. Innov. 17, 100592 (2020). https://doi.org/10.1016/j.eti.2019.100592. [Google Scholar]
- G.H. Wang, J.S. Liu, X.G. Wang, Z.Y. Xie, N.S. Deng, Sorption behavior of U(VI) onto Chinese bentonite: Effect of pH, ionic strength, temperature and humic acid. J. Molecular Liq. 295, 1927–1934 (2013). https://doi.org/10.1016/j.molliq.2013.10.008. [Google Scholar]
- Y.G. Chen, Z. Sun, W.M. Ye, Adsorptive removal of Eu(III) from simulated groundwater by GMZ bentonite on the repository conditions. J. Radioanal. Nucl. Chem. 311, 1839–1847 (2017). https://doi.org/10.1007/s10967-017-5173-6. [Google Scholar]
- W.A. Muslim, et al., Investigation of bentonite clay minerals as natural adsorbents for Cs-137 real radioactive wastewater treatment. Desal Water Treat. 317, 100121 (2024). https://doi.org/10.1016/j.dwt.2024.100121. [Google Scholar]
- W.A. Muslim, S.K. Al-Nasri, T.M. Albayati, Evaluation of bentonite, attapulgite, and kaolinite as eco-friendly adsorbents in the treatment of real radioactive wastewater containing Cs-137. Prog Nucl. Energy 165, 104730 (2023). https://doi.org/10.1016/j.pnucene.2023.104730. [Google Scholar]
- D. Yılmaz, A. Gürses, S. Kalecik, A. Maman, E. Şahin, K. Güneş, Removal of 177Lu from radioactive wastewater using Montmorillonite clay. Appl. Radiat. Isot. 211, 111417 (2024). https://doi.org/10.1016/j.apradiso.2024.111417. [Google Scholar]
- T. Takehiko, Adsorption of uranium from acidic solution by microbes and effect of thorium on uranium adsorption by Streptomyces levoris. J. Biosci. Bioeng. 97, 4, 275–277 (2004). https://doi.org/10.1016/S1389-1723(04)70203-0. [Google Scholar]
- J.L. Liao, et al., Method for treating 137Cs radioactive wastewater using microbial adsorption (2017). CN103755036B. https://patents.google.com/patent/CN103755036B/en. [Google Scholar]
- I.B. Rae, S. Pap, D. Svobodova, S.W. Gibb, Comparison of sustainable biosorbents and ion-exchange resins to remove Sr2+ from simulant nuclear wastewater: Batch, dynamic and mechanism studies. Sci. Total Environ. 650, 2411–2422 (2019). https://doi.org/10.1016/j.scitotenv.2018.09.396. [Google Scholar]
- A. Benalia, et al., Use of extracted proteins from oak leaves as bio-coagulant for water and wastewater treatment: Optimization by a fractional factorial design. Water 15(11), 1984 (2023). https://doi.org/10.3390/w15111984. [Google Scholar]
- M. Sharma, A. Anshika, L. Yadav, P. Sharma, V.C. Janu, R. Gupta, Breaking new ground: Innovative adsorbents for uranium and thorium ions removal and environmental cleanup. Coord. Chem. Rev. 517, 216008 (2024). https://doi.org/10.1016/j.ccr.2024.216008. [Google Scholar]
- V. Sodha, S. Shahabuddin, R. Gaur, I. Ahmad, R. Bandyopadhyay, N. Sridewi, Comprehensive review on zeolite-based nanocomposites for treatment of effluents from wastewater. Nanomaterials 12(18), 3199 (2022). https://doi.org/10.3390/nano12183199. [Google Scholar]
- K. Li, et al., High efficiency removal of Cs+ by Fe-Co framework PBAs from radioactive wastewater. J. Environ. Chem. Eng. 12(6), 114314 (2024). https://doi.org/10.1016/j.jece.2024.114314. [Google Scholar]
- X. Chen, L. Wang, C. Ding, H. Xie, H. Zou, J. Deng, Z. Liu, J. Shi, Y. Ding, Highly efficient removal of radioactive iodine anions by nano silver modified activated carbon fiber. Appl. Surf. Sci. 643, 158644 (2024). https://doi.org/10.1016/j.apsusc.2023.158644. [Google Scholar]
- Y. Meng, Y. Wang, Z. Ye, N. Wang, C. He, Y. Zhu, T. Fujita, H. Wu, X. Wang, Three-dimension titanium phosphate aerogel for selective removal of radioactive strontium(II) from contaminated waters. J. Environ. Manag. 325, 116424 (2023). https://doi.org/10.1016/j.jenvman.2022.116424. [Google Scholar]
- E. Cho, J. Kim, C.W. Park, K.-W. Lee, T.S. Lee, Chemically bound Prussian blue in sodium alginate hydrogel for enhanced removal of Cs ions. J. Hazard. Mater. 360, 243–249 (2018). https://doi.org/10.1016/j.jhazmat.2018.08.031. [Google Scholar]
- Y. Hu, et al., Ultra-fast adsorption of radioactive-U(VI) and Cs(I) with high adsorption capacity towards CAA@MgAlFe spongy-like aerogel: Mechanism and application feasibility study. J. Nucl. Mater. 559, 153463 (2022). https://doi.org/10.1016/j.jnucmat.2021.153463. [Google Scholar]
- Q.H. Ye, H. Ye, Y.C. Yu, X.L. Wang, Q. Luo, M.B. Wu, J. Yao, Interface-confined nanocatalysts at hollow porous nanofibers for high-performance cascaded remediation of radioactive wastewater. Chem. Eng. J. 500, 157170 (2024). https://doi.org/10.1016/j.cej.2024.157170 . [Google Scholar]
- Y. Ding, et al., Rapid one-step preparation of a carboxymethyl chitosan gel with a novel crosslinker for efficient adsorption of Sr2+. Colloids Surf. A Physicochem. Eng. Asp. 641, 128576 (2022). https://doi.org/10.1016/j.colsurfa.2022.128576. [Google Scholar]
- S. Zhuang, K. Zhu, L. Xu, J. Hu, J. Wang, Adsorption of Co2+ and Sr2+ in aqueous solution by a novel fibrous chitosan biosorbent. Sci. Total Environ. 825, 153998 (2022). https://doi.org/10.1016/j.scitotenv.2022.153998. [Google Scholar]
- L. Guo, et al., Construction of novel phytic acid-based lignin for highly efficient treatment of low-level radioactive wastewater: Synthesis, performance, and mechanistic insights. Sep. Purif. Technol. 341, 126969 (2024). https://doi.org/10.1016/j.seppur.2024.126969. [Google Scholar]
- Y.X. Zheng, et al., Nitrogen-rich and core-sheath polyamide/polyethyleneimine@Zr-MOF for iodine adsorption and nerve agent simulant degradation. J. Hazard. Mater. 480, 135713 (2024). https://doi.org/10.1016/j.jhazmat.2024.135713. [Google Scholar]
- Y. Li, T. Pan, J. Feng, B. Yu, W. Xiong, G. Yuan, Facile preparation of UiO-66-Lys/PAN nanofiber membrane by electrospinning for the removal of Co(II) from simulated radioactive wastewater. Sci. Total Environ. 914, 169725 (2024). https://doi.org/10.1016/j.scitotenv.2023.169725. [Google Scholar]
-
S. Yang, J. Yin, Q. Li, C. Wang, D. Hua, N. Wu, Covalent organic frameworks functionalized electrodes for simultaneous removal of
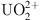 and
and 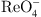 with fast kinetics and high capacities by electro-adsorption. J. Hazard. Mater. 429, 128315 (2022). https://doi.org/10.1016/j.jhazmat.2022.128315.
[Google Scholar]
with fast kinetics and high capacities by electro-adsorption. J. Hazard. Mater. 429, 128315 (2022). https://doi.org/10.1016/j.jhazmat.2022.128315.
[Google Scholar]
- R. Li, F. Sun, Z. Liu, Y. Shi, S. He, J. Chen, Research progress and prospect of covalent organic frameworks (COFs) and composites: From synthesis to application in water contaminants. J. Environ. Chem. Eng. 12(5), 113944 (2024). https://doi.org/10.1016/j.jece.2024.113944. [Google Scholar]
- Y.-L. Liu, D. Li, P. Cao, Advances in MXene-based composite materials for efficient removal of radioactive nuclides and heavy metal ions. Mater. Today Phys. 44, 101444 (2024). https://doi.org/10.1016/j.mtphys.2024.101444. [Google Scholar]
- J. Yan, H.J. Liu, L. Xie, Z. Liu, P.F. Liu, H.X. Wen, Europium(III) removal from aqueous solution using citric acid modified alkalized Mxene as an adsorbent. J. Radioanal. Nucl. Chem. 331(2), 1063–1073 (2022). https://doi.org/10.1007/s10967-021-08154-4. [Google Scholar]
- M. Li, X. Tang, J. Tan, G. Cheng, F. Wu, N. Zhou, Properties and mechanism of uranium adsorption on single-sided fluorinated graphene: A first-principles study. Surf. Interfaces 50, 104504 (2024). https://doi.org/10.1016/j.surfin.2024.104504. [Google Scholar]
- K. Shao, et al., Efficient removal of U(VI) by graphene-based electrode modified with amidoxime: Performance and mechanism. Sep. Purif. Technol. 354, 128822 (2025). https://doi.org/10.1016/j.seppur.2024.128822. [Google Scholar]
- S. Zhuang, Y. Mei, J. Wang, Adsorption performance and mechanisms of Co2+ onto carboxyl-functionalized carbon nanotubes. J. Clean. Prod. 430, 139709 (2023). https://doi.org/10.1016/j.jclepro.2023.139709. [Google Scholar]
- J. Zhang, Y. Wang, Y. Wei, M. Xu, J. Li, Magnetic CNT-based electrode for efficient electro-adsorption of uranium. J. Environ. Chem. Eng. 12(2), 112160 (2024). https://doi.org/10.1016/j.jece.2024.112160. [Google Scholar]
- H. Chaudhuri, Y.-S. Yun, Synthesis and environmental applications of graphene oxide/layered double hydroxides and graphene oxide/MXenes: A critical review. Sep. Purif. Technol. 297, 121518 (2022). https://doi.org/10.1016/j.seppur.2022.121518. [Google Scholar]
- X. Long, Y.-S. Chen, Q. Zheng, X.-X. Xie, H. Tang, L.-P. Jiang, J.-T. Jiang, J.-H. Qiu, Removal of iodine from aqueous solution by PVDF/ZIF-8 nanocomposite membranes. Sep. Purif. Technol. 238, 116488 (2020). https://doi.org/10.1016/j.seppur.2019.116488. [Google Scholar]
- C.Y. Chen, et al., Preparation of a microfilm adsorbent material for rapid and efficient adsorption of radionuclide thorium ions (China Patent CN202310499379.4, filed May 6, 2023, 2023). [Google Scholar]
- H. Xiao, et al., In situ growth of two-dimensional ZIF-L nanoflakes on ceramic membrane for efficient removal of iodine. J. Membr. Sci. 619, 118782 (2020). https://doi.org/10.1016/j.memsci.2020.118709. [Google Scholar]
- L.Y. Xu, Q. Zheng, Y.Y. Wang, L. Jiang, J. Jiang, J.H. Qiu, A pillared double-wall metal-organic framework adsorption membrane for the efficient removal of iodine from solution. Sep. Purif. Technol. 274, 118436 (2021). https://doi.org/10.1016/j.seppur.2021.118436. [Google Scholar]
- J.Y. Cheng, K.L. Liu, X. Li, L. Huang, J. Liang, G.P. Zheng, G.C. Shan, Nickel–metal–organic framework nanobelt-based composite membranes for efficient Sr2+ removal from aqueous solution. Environ. Sci. Ecol. Technol. 3, 100035 (2020). https://doi.org/10.1016/j.ese.2020.100035. [Google Scholar]
- F.F. Gao, J.H. Luo, X.F. Zhang, X.G. Hao, G.Q. Guan, Z. Liu, J. Li, Q.L. Luo, Electrodeposited iodide ions imprinted polypyrrole@bismuth oxyiodide film for an electrochemically switched renewable extractor towards iodide ions. Chin. J. Chem. Eng. 49, 161–169 (2022). https://doi.org/10.1016/j.cjche.2022.05.014. [Google Scholar]
- J.H. Luo, X. Du, F.F. Gao, P.F. Ma, X.G. Hao, G.Q. Guan, O. Scialdone, J. Li, Electrochemically triggered iodide-vacancy BiOI film for selective extraction of iodide ion from aqueous solutions. Sep. Purif. Technol. 259, 118120 (2021). https://doi.org/10.1016/j.seppur.2020.118120. [Google Scholar]
- H. Zhang, Y.J. Chi, J.Y. Li, J.S. Peng, H.Y. Song, C.X. Chen, X.F. Bai, Enhanced adsorption of radioactive cesium from nuclear wastewater using ZIF-67 laminated 2D MXene Ti3C2. Sep. Purif. Technol. 355(A), 129590 (2025). https://doi.org/10.1016/j.seppur.2024.129590. [Google Scholar]
- W.A. Muslim, T.M. Albayati, S.K. Al-Nasri, K.T. Rashid, I.K. Salih, A.S. Al-Nasri, A hybrid adsorption/ultrafiltration membrane process for removal of Cs-137 from radioactive wastewater using natural clay adsorbent. Chem. Eng. Res. Des. 208, 853–862 (2024). https://doi.org/10.1016/j.cherd.2024.07.036. [Google Scholar]
- Y.Q. Li, T. Pan, J. Feng, B. Yu, W. Xiong, G.Y. Yuan, Facile preparation of UiO-66-Lys/PAN nanofiber membrane by electrospinning for the removal of Co(II) from simulated radioactive wastewater. Sci. Total Environ. 914, 169725 (2024). https://doi.org/10.1016/j.scitotenv.2023.169725. [Google Scholar]
- G.Y. Yuan, Y.Q. Liu, Y.Q. Li, Y.Y. Yu, Y.L. Lei, F. Liu, L. Fan, D.R. Liu, X.Q. Pu, W. Xiong, Facile construction of a core-shell structured metal-organic frameworks nanofiber membrane for removing Co(II) from simulated radioactive wastewater. Sep. Purif. Technol. 336, 126295 (2024). https://doi.org/10.1016/j.seppur.2024.126295. [Google Scholar]
- K. Yu, P. Shao, P. Meng, T. Chen, J. Lei, X. Yu, R. He, F. Yang, W. Zhu, T. Duan, Superhydrophilic and highly elastic monolithic sponge for efficient solar-driven radioactive wastewater treatment under one sun. J. Hazard. Mater. 392, 122350 (2020). https://doi.org/10.1016/j.jhazmat.2020.122350. [Google Scholar]
Cite this article as: Gao J, Lv A, Zhao Z, Luo X, Li Z, et al. Research advances on adsorption materials for the purification of radioactive wastewater, Res. Des. Nucl. Eng. 1, 2025003 (2025) https://doi.org/10.1051/rdne/2025003.
All Tables
Performance and characteristics of natural adsorption materials applied in radioactive wastewater purification.
Performance and characteristics of composite adsorption materials applied in radioactive wastewater purification.
Performance and characteristics of engineered adsorption materials applied in radioactive wastewater purification.
Summary of functional groups, adsorption mechanisms, and representative materials for various radioactive nuclides.
Performance and characteristics of adsorption membrane materials applied in radioactive wastewater purification.
All Figures
 |
Fig. 1 Annual number of publications on radioactive wastewater treatment using adsorption, membrane technologies, electrochemical methods, evaporation systems, phytoremediation, and precipitation from 2014 to 2024. Data were obtained from the Web of Science Core Collection. |
| In the text | |
 |
Fig. 2 (a) Main adsorption mechanisms of bentonite-based materials for radionuclides [11]; (b) (1): Adsorption capacity (qe) (2): Adsorption distribution coefficient (Kd), A: Attapulgite, B: Bentonite, K: Kaolinite [13]; (c) Cross-sectional model of montmorillonite layered silicate sheets [11]. |
| In the text | |
 |
Fig. 3 Schematic diagram of the preparation process for CAA@MgAlFe [25]. |
| In the text | |
 |
Fig. 4 Adsorption mechanism diagram of CAA@MgAlFe [25]. |
| In the text | |
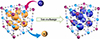 |
Fig. 5 Schematic diagram of the Cs+ adsorption mechanism by PBAFe [21]. |
| In the text | |
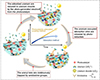 |
Fig. 6 Schematic diagram of the uranium removal process using HPN [26]. |
| In the text | |
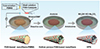 |
Fig. 7 Schematic diagram of the HPN preparation process [26]. |
| In the text | |
 |
Fig. 8 Dual functionality of PPFM composites in the treatment of toxic compounds [30]. |
| In the text | |
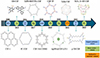 |
Fig. 9 Development history of covalent organic frameworks (COFs) [33]. |
| In the text | |
 |
Fig. 10 Synthesis process and structural characteristics of MXene [34]. |
| In the text | |
 |
Fig. 11 Structure of TiO2/MXene composites [34]. |
| In the text | |
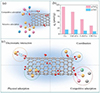 |
Fig. 12 Comparison of adsorption capacities of cCNT and fCNT for different metal ions [38]. |
| In the text | |
 |
Fig. 13 Comparison of equilibrium adsorption capacities of Sr2+ among natural, composite, and engineered adsorbents. Data were obtained from the Web of Science Core Collection. |
| In the text | |
 |
Fig. 14 Preparation process of PVDF/ZIF-8 nanocomposite membrane and schematic diagram of iodine removal from water [41]. |
| In the text | |
 |
Fig. 15 Schematic diagram of the preparation process for ZIF-L@SiC membrane [43]. |
| In the text | |
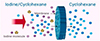 |
Fig. 16 Schematic diagram of iodine adsorption process by ZIF-L@SiC membrane [43]. |
| In the text | |
 |
Fig. 17 Schematic diagram of the preparation process and dynamic adsorption process of lac-Zn columnar double-walled MOF adsorption membrane [44]. |
| In the text | |
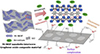 |
Fig. 18 Schematic diagram of the adsorption mechanism of GO/Ni-MOF for Sr2O3 [45]. |
| In the text | |
 |
Fig. 19 Schematic diagram of iodine vacancy BiOI film for adsorption and extraction of iodine ions from solutions containing radioactive elements [47]. |
| In the text | |
 |
Fig. 20 (a) Schematic diagram of the preparation process of KGS; (b–d) SEM images of KGS porous structures with different cations [52]. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

