Open Access
Review
| Issue |
Res. Des. Nucl. Eng.
Volume 1, 2025
|
|
|---|---|---|
| Article Number | 2025003 | |
| Number of page(s) | 19 | |
| DOI | https://doi.org/10.1051/rdne/2025003 | |
| Published online | 04 July 2025 | |
- H. Ma, M. Shen, Y. Tong, X. Wang, Radioactive wastewater treatment technologies: a review. Molecules 28, 1935 (2023). https://doi.org/10.3390/molecules28041935. [Google Scholar]
- H. Liu, T. Fu, M.T. Sarwar, H. Yang, Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. Appl. Clay Sci. 232, 106796 (2023). https://doi.org/10.1016/j.clay.2022.106796. [Google Scholar]
- D. Kadadou, E.A. Said, R. Ajaj, S.W. Hasan, Research advances in nuclear wastewater treatment using conventional and hybrid technologies: Towards sustainable wastewater reuse and recovery. J. Water Process Eng. 52, 103604 (2023). https://doi.org/10.1016/j.jwpe.2023.103604. [Google Scholar]
- G.D. Yuan, et al., Clays and clay minerals for pollution control. Develop. Clay Sci. 5, 587–644 (2013). [Google Scholar]
- W.A. Muslim, S.K. Al-Nasri, T.M. Albayati, H.Sh. Majdi, Treatment of actual radioactive wastewater containing Cs-137 using kaolinite clay minerals as eco-friendly adsorbents. Desal. Water Treat. 307, 162–170 (2023). https://doi.org/10.5004/dwt.2023.29908. [Google Scholar]
- O. Pourret, A. Hursthouse, It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int. J. Environ. Res. Public Health 16, 4446 (2019). https://doi.org/10.3390/ijerph16224446. [Google Scholar]
- A.M. El-Kamash, Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater. 151, 432–445 (2008). https://doi.org/10.1016/j.jhazmat.2007.06.009. [Google Scholar]
- I. Liaquat, R. Munir, N.A. Abbasi, B. Sadia, A. Muneer, F. Younas, M.F. Sardar, M. Zahid, S. Noreen, Exploring zeolite-based composites in adsorption and photocatalysis for toxic wastewater treatment: Preparation, mechanisms, and future perspectives. Environ. Pollut. 349, 123922 (2024). https://doi.org/10.1016/j.envpol.2024.123922. [Google Scholar]
- T. Abdollahi, J. Towfighi, H. Rezaei-Vahidian, Sorption of cesium and strontium ions by natural zeolite and management of produced secondary waste. Environ. Technol. Innov. 17, 100592 (2020). https://doi.org/10.1016/j.eti.2019.100592. [Google Scholar]
- G.H. Wang, J.S. Liu, X.G. Wang, Z.Y. Xie, N.S. Deng, Sorption behavior of U(VI) onto Chinese bentonite: Effect of pH, ionic strength, temperature and humic acid. J. Molecular Liq. 295, 1927–1934 (2013). https://doi.org/10.1016/j.molliq.2013.10.008. [Google Scholar]
- Y.G. Chen, Z. Sun, W.M. Ye, Adsorptive removal of Eu(III) from simulated groundwater by GMZ bentonite on the repository conditions. J. Radioanal. Nucl. Chem. 311, 1839–1847 (2017). https://doi.org/10.1007/s10967-017-5173-6. [Google Scholar]
- W.A. Muslim, et al., Investigation of bentonite clay minerals as natural adsorbents for Cs-137 real radioactive wastewater treatment. Desal Water Treat. 317, 100121 (2024). https://doi.org/10.1016/j.dwt.2024.100121. [Google Scholar]
- W.A. Muslim, S.K. Al-Nasri, T.M. Albayati, Evaluation of bentonite, attapulgite, and kaolinite as eco-friendly adsorbents in the treatment of real radioactive wastewater containing Cs-137. Prog Nucl. Energy 165, 104730 (2023). https://doi.org/10.1016/j.pnucene.2023.104730. [Google Scholar]
- D. Yılmaz, A. Gürses, S. Kalecik, A. Maman, E. Şahin, K. Güneş, Removal of 177Lu from radioactive wastewater using Montmorillonite clay. Appl. Radiat. Isot. 211, 111417 (2024). https://doi.org/10.1016/j.apradiso.2024.111417. [Google Scholar]
- T. Takehiko, Adsorption of uranium from acidic solution by microbes and effect of thorium on uranium adsorption by Streptomyces levoris. J. Biosci. Bioeng. 97, 4, 275–277 (2004). https://doi.org/10.1016/S1389-1723(04)70203-0. [Google Scholar]
- J.L. Liao, et al., Method for treating 137Cs radioactive wastewater using microbial adsorption (2017). CN103755036B. https://patents.google.com/patent/CN103755036B/en. [Google Scholar]
- I.B. Rae, S. Pap, D. Svobodova, S.W. Gibb, Comparison of sustainable biosorbents and ion-exchange resins to remove Sr2+ from simulant nuclear wastewater: Batch, dynamic and mechanism studies. Sci. Total Environ. 650, 2411–2422 (2019). https://doi.org/10.1016/j.scitotenv.2018.09.396. [Google Scholar]
- A. Benalia, et al., Use of extracted proteins from oak leaves as bio-coagulant for water and wastewater treatment: Optimization by a fractional factorial design. Water 15(11), 1984 (2023). https://doi.org/10.3390/w15111984. [Google Scholar]
- M. Sharma, A. Anshika, L. Yadav, P. Sharma, V.C. Janu, R. Gupta, Breaking new ground: Innovative adsorbents for uranium and thorium ions removal and environmental cleanup. Coord. Chem. Rev. 517, 216008 (2024). https://doi.org/10.1016/j.ccr.2024.216008. [Google Scholar]
- V. Sodha, S. Shahabuddin, R. Gaur, I. Ahmad, R. Bandyopadhyay, N. Sridewi, Comprehensive review on zeolite-based nanocomposites for treatment of effluents from wastewater. Nanomaterials 12(18), 3199 (2022). https://doi.org/10.3390/nano12183199. [Google Scholar]
- K. Li, et al., High efficiency removal of Cs+ by Fe-Co framework PBAs from radioactive wastewater. J. Environ. Chem. Eng. 12(6), 114314 (2024). https://doi.org/10.1016/j.jece.2024.114314. [Google Scholar]
- X. Chen, L. Wang, C. Ding, H. Xie, H. Zou, J. Deng, Z. Liu, J. Shi, Y. Ding, Highly efficient removal of radioactive iodine anions by nano silver modified activated carbon fiber. Appl. Surf. Sci. 643, 158644 (2024). https://doi.org/10.1016/j.apsusc.2023.158644. [Google Scholar]
- Y. Meng, Y. Wang, Z. Ye, N. Wang, C. He, Y. Zhu, T. Fujita, H. Wu, X. Wang, Three-dimension titanium phosphate aerogel for selective removal of radioactive strontium(II) from contaminated waters. J. Environ. Manag. 325, 116424 (2023). https://doi.org/10.1016/j.jenvman.2022.116424. [Google Scholar]
- E. Cho, J. Kim, C.W. Park, K.-W. Lee, T.S. Lee, Chemically bound Prussian blue in sodium alginate hydrogel for enhanced removal of Cs ions. J. Hazard. Mater. 360, 243–249 (2018). https://doi.org/10.1016/j.jhazmat.2018.08.031. [Google Scholar]
- Y. Hu, et al., Ultra-fast adsorption of radioactive-U(VI) and Cs(I) with high adsorption capacity towards CAA@MgAlFe spongy-like aerogel: Mechanism and application feasibility study. J. Nucl. Mater. 559, 153463 (2022). https://doi.org/10.1016/j.jnucmat.2021.153463. [Google Scholar]
- Q.H. Ye, H. Ye, Y.C. Yu, X.L. Wang, Q. Luo, M.B. Wu, J. Yao, Interface-confined nanocatalysts at hollow porous nanofibers for high-performance cascaded remediation of radioactive wastewater. Chem. Eng. J. 500, 157170 (2024). https://doi.org/10.1016/j.cej.2024.157170 . [Google Scholar]
- Y. Ding, et al., Rapid one-step preparation of a carboxymethyl chitosan gel with a novel crosslinker for efficient adsorption of Sr2+. Colloids Surf. A Physicochem. Eng. Asp. 641, 128576 (2022). https://doi.org/10.1016/j.colsurfa.2022.128576. [Google Scholar]
- S. Zhuang, K. Zhu, L. Xu, J. Hu, J. Wang, Adsorption of Co2+ and Sr2+ in aqueous solution by a novel fibrous chitosan biosorbent. Sci. Total Environ. 825, 153998 (2022). https://doi.org/10.1016/j.scitotenv.2022.153998. [Google Scholar]
- L. Guo, et al., Construction of novel phytic acid-based lignin for highly efficient treatment of low-level radioactive wastewater: Synthesis, performance, and mechanistic insights. Sep. Purif. Technol. 341, 126969 (2024). https://doi.org/10.1016/j.seppur.2024.126969. [Google Scholar]
- Y.X. Zheng, et al., Nitrogen-rich and core-sheath polyamide/polyethyleneimine@Zr-MOF for iodine adsorption and nerve agent simulant degradation. J. Hazard. Mater. 480, 135713 (2024). https://doi.org/10.1016/j.jhazmat.2024.135713. [Google Scholar]
- Y. Li, T. Pan, J. Feng, B. Yu, W. Xiong, G. Yuan, Facile preparation of UiO-66-Lys/PAN nanofiber membrane by electrospinning for the removal of Co(II) from simulated radioactive wastewater. Sci. Total Environ. 914, 169725 (2024). https://doi.org/10.1016/j.scitotenv.2023.169725. [Google Scholar]
-
S. Yang, J. Yin, Q. Li, C. Wang, D. Hua, N. Wu, Covalent organic frameworks functionalized electrodes for simultaneous removal of
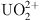 and
and 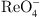 with fast kinetics and high capacities by electro-adsorption. J. Hazard. Mater. 429, 128315 (2022). https://doi.org/10.1016/j.jhazmat.2022.128315.
[Google Scholar]
with fast kinetics and high capacities by electro-adsorption. J. Hazard. Mater. 429, 128315 (2022). https://doi.org/10.1016/j.jhazmat.2022.128315.
[Google Scholar]
- R. Li, F. Sun, Z. Liu, Y. Shi, S. He, J. Chen, Research progress and prospect of covalent organic frameworks (COFs) and composites: From synthesis to application in water contaminants. J. Environ. Chem. Eng. 12(5), 113944 (2024). https://doi.org/10.1016/j.jece.2024.113944. [Google Scholar]
- Y.-L. Liu, D. Li, P. Cao, Advances in MXene-based composite materials for efficient removal of radioactive nuclides and heavy metal ions. Mater. Today Phys. 44, 101444 (2024). https://doi.org/10.1016/j.mtphys.2024.101444. [Google Scholar]
- J. Yan, H.J. Liu, L. Xie, Z. Liu, P.F. Liu, H.X. Wen, Europium(III) removal from aqueous solution using citric acid modified alkalized Mxene as an adsorbent. J. Radioanal. Nucl. Chem. 331(2), 1063–1073 (2022). https://doi.org/10.1007/s10967-021-08154-4. [Google Scholar]
- M. Li, X. Tang, J. Tan, G. Cheng, F. Wu, N. Zhou, Properties and mechanism of uranium adsorption on single-sided fluorinated graphene: A first-principles study. Surf. Interfaces 50, 104504 (2024). https://doi.org/10.1016/j.surfin.2024.104504. [Google Scholar]
- K. Shao, et al., Efficient removal of U(VI) by graphene-based electrode modified with amidoxime: Performance and mechanism. Sep. Purif. Technol. 354, 128822 (2025). https://doi.org/10.1016/j.seppur.2024.128822. [Google Scholar]
- S. Zhuang, Y. Mei, J. Wang, Adsorption performance and mechanisms of Co2+ onto carboxyl-functionalized carbon nanotubes. J. Clean. Prod. 430, 139709 (2023). https://doi.org/10.1016/j.jclepro.2023.139709. [Google Scholar]
- J. Zhang, Y. Wang, Y. Wei, M. Xu, J. Li, Magnetic CNT-based electrode for efficient electro-adsorption of uranium. J. Environ. Chem. Eng. 12(2), 112160 (2024). https://doi.org/10.1016/j.jece.2024.112160. [Google Scholar]
- H. Chaudhuri, Y.-S. Yun, Synthesis and environmental applications of graphene oxide/layered double hydroxides and graphene oxide/MXenes: A critical review. Sep. Purif. Technol. 297, 121518 (2022). https://doi.org/10.1016/j.seppur.2022.121518. [Google Scholar]
- X. Long, Y.-S. Chen, Q. Zheng, X.-X. Xie, H. Tang, L.-P. Jiang, J.-T. Jiang, J.-H. Qiu, Removal of iodine from aqueous solution by PVDF/ZIF-8 nanocomposite membranes. Sep. Purif. Technol. 238, 116488 (2020). https://doi.org/10.1016/j.seppur.2019.116488. [Google Scholar]
- C.Y. Chen, et al., Preparation of a microfilm adsorbent material for rapid and efficient adsorption of radionuclide thorium ions (China Patent CN202310499379.4, filed May 6, 2023, 2023). [Google Scholar]
- H. Xiao, et al., In situ growth of two-dimensional ZIF-L nanoflakes on ceramic membrane for efficient removal of iodine. J. Membr. Sci. 619, 118782 (2020). https://doi.org/10.1016/j.memsci.2020.118709. [Google Scholar]
- L.Y. Xu, Q. Zheng, Y.Y. Wang, L. Jiang, J. Jiang, J.H. Qiu, A pillared double-wall metal-organic framework adsorption membrane for the efficient removal of iodine from solution. Sep. Purif. Technol. 274, 118436 (2021). https://doi.org/10.1016/j.seppur.2021.118436. [Google Scholar]
- J.Y. Cheng, K.L. Liu, X. Li, L. Huang, J. Liang, G.P. Zheng, G.C. Shan, Nickel–metal–organic framework nanobelt-based composite membranes for efficient Sr2+ removal from aqueous solution. Environ. Sci. Ecol. Technol. 3, 100035 (2020). https://doi.org/10.1016/j.ese.2020.100035. [Google Scholar]
- F.F. Gao, J.H. Luo, X.F. Zhang, X.G. Hao, G.Q. Guan, Z. Liu, J. Li, Q.L. Luo, Electrodeposited iodide ions imprinted polypyrrole@bismuth oxyiodide film for an electrochemically switched renewable extractor towards iodide ions. Chin. J. Chem. Eng. 49, 161–169 (2022). https://doi.org/10.1016/j.cjche.2022.05.014. [Google Scholar]
- J.H. Luo, X. Du, F.F. Gao, P.F. Ma, X.G. Hao, G.Q. Guan, O. Scialdone, J. Li, Electrochemically triggered iodide-vacancy BiOI film for selective extraction of iodide ion from aqueous solutions. Sep. Purif. Technol. 259, 118120 (2021). https://doi.org/10.1016/j.seppur.2020.118120. [Google Scholar]
- H. Zhang, Y.J. Chi, J.Y. Li, J.S. Peng, H.Y. Song, C.X. Chen, X.F. Bai, Enhanced adsorption of radioactive cesium from nuclear wastewater using ZIF-67 laminated 2D MXene Ti3C2. Sep. Purif. Technol. 355(A), 129590 (2025). https://doi.org/10.1016/j.seppur.2024.129590. [Google Scholar]
- W.A. Muslim, T.M. Albayati, S.K. Al-Nasri, K.T. Rashid, I.K. Salih, A.S. Al-Nasri, A hybrid adsorption/ultrafiltration membrane process for removal of Cs-137 from radioactive wastewater using natural clay adsorbent. Chem. Eng. Res. Des. 208, 853–862 (2024). https://doi.org/10.1016/j.cherd.2024.07.036. [Google Scholar]
- Y.Q. Li, T. Pan, J. Feng, B. Yu, W. Xiong, G.Y. Yuan, Facile preparation of UiO-66-Lys/PAN nanofiber membrane by electrospinning for the removal of Co(II) from simulated radioactive wastewater. Sci. Total Environ. 914, 169725 (2024). https://doi.org/10.1016/j.scitotenv.2023.169725. [Google Scholar]
- G.Y. Yuan, Y.Q. Liu, Y.Q. Li, Y.Y. Yu, Y.L. Lei, F. Liu, L. Fan, D.R. Liu, X.Q. Pu, W. Xiong, Facile construction of a core-shell structured metal-organic frameworks nanofiber membrane for removing Co(II) from simulated radioactive wastewater. Sep. Purif. Technol. 336, 126295 (2024). https://doi.org/10.1016/j.seppur.2024.126295. [Google Scholar]
- K. Yu, P. Shao, P. Meng, T. Chen, J. Lei, X. Yu, R. He, F. Yang, W. Zhu, T. Duan, Superhydrophilic and highly elastic monolithic sponge for efficient solar-driven radioactive wastewater treatment under one sun. J. Hazard. Mater. 392, 122350 (2020). https://doi.org/10.1016/j.jhazmat.2020.122350. [Google Scholar]
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.

